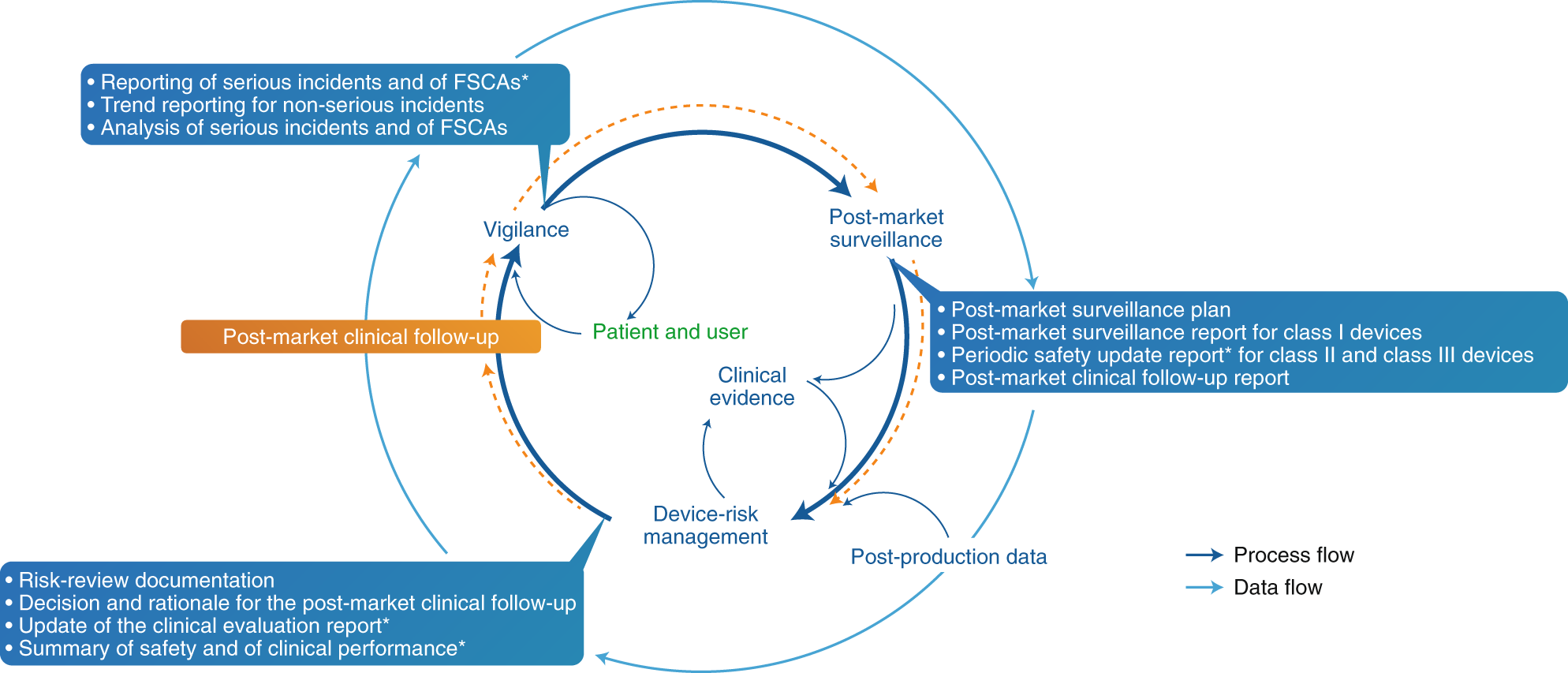
How the new European regulation on medical devices will affect innovation | Nature Biomedical Engineering

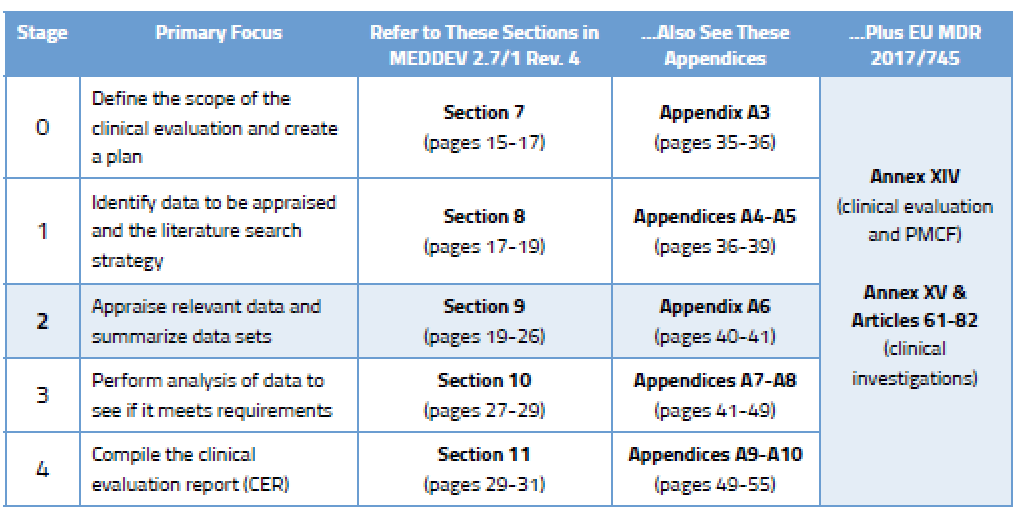
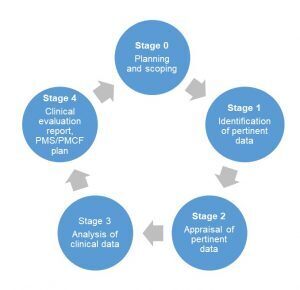
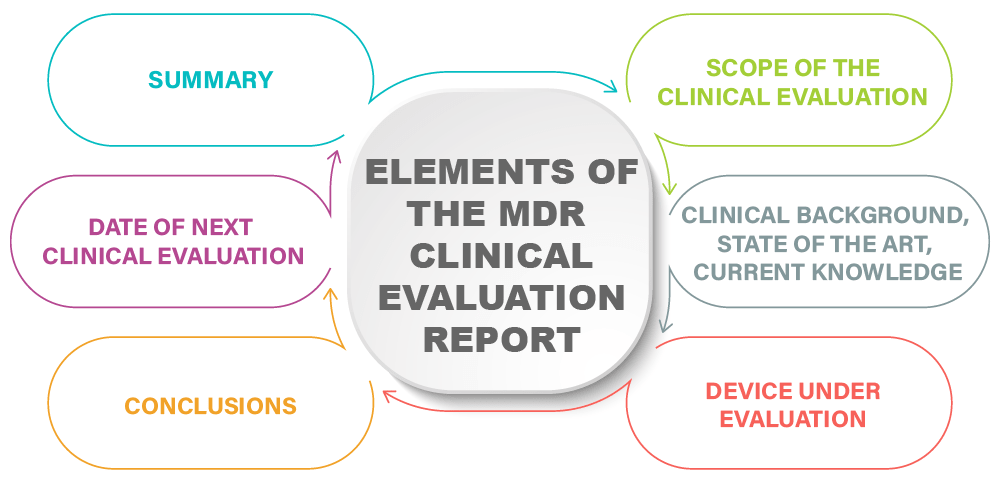
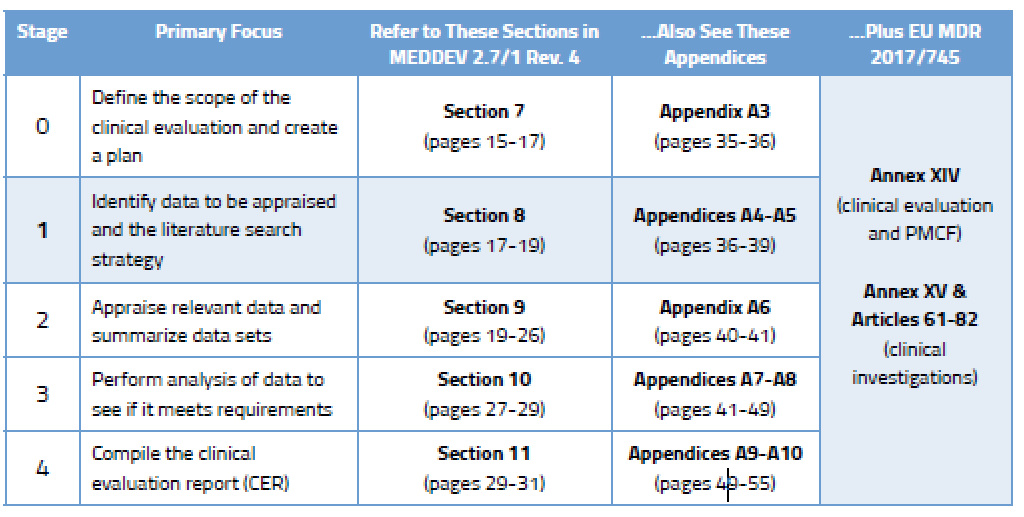
How to perform a clinical evaluation of medical devices – Part 1 – Overview and sample of activities – Medical Device Expert News

The medical device challenge in Europe (Part XV): Why conducting a full clinical evaluation now- to meet MDR requirements - is a very good idea

Importance of systematic literature search for clinical evaluation(ce) the strict adherence of medde by PepGra CRO - Issuu

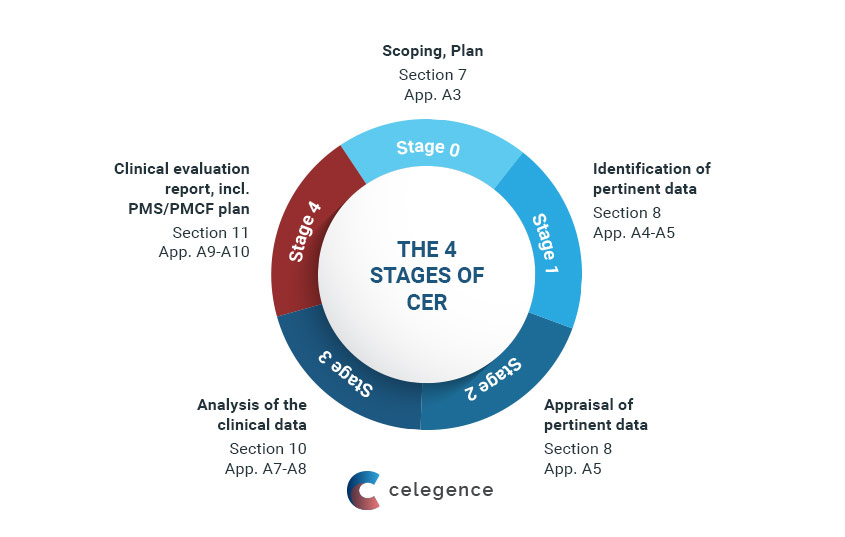


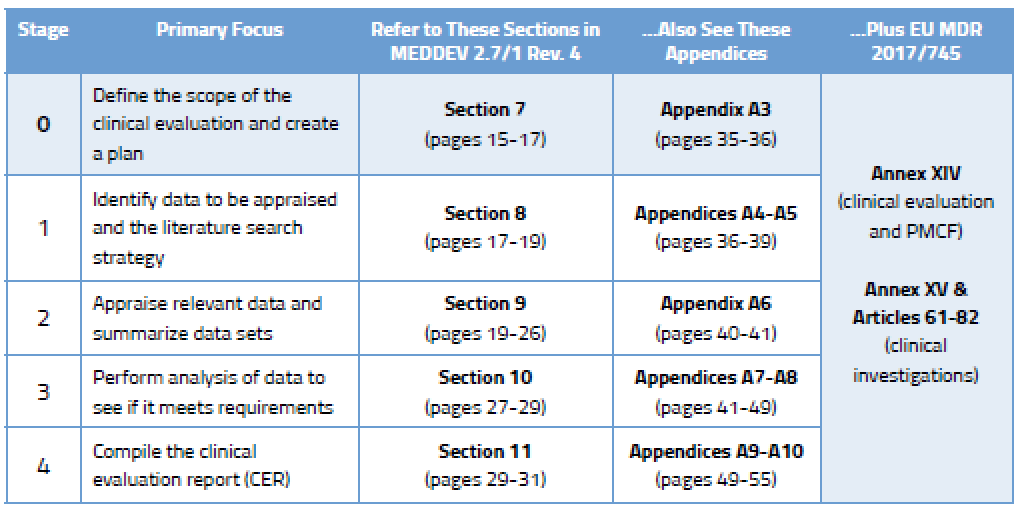



.png.aspx)