PDF) Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis
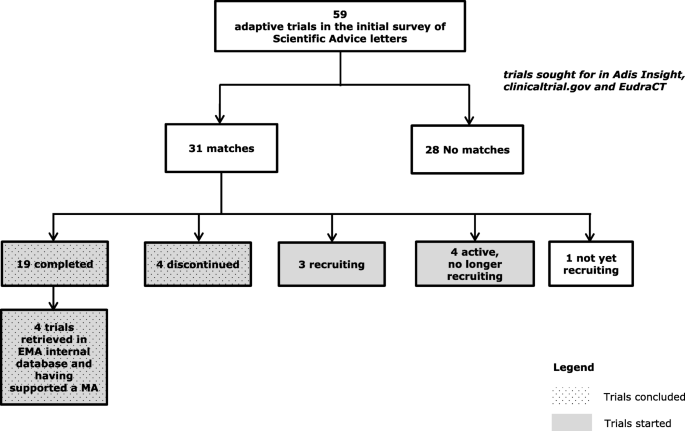
Adaptive designs in clinical trials: from scientific advice to marketing authorisation to the European Medicine Agency | Trials | Full Text

Risk of bias in industry-funded oseltamivir trials: comparison of core reports versus full clinical study reports | BMJ Open

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH
The European Medicines Agency Clinical Data Website Enables Insights Into Clinical Development Timelines And Strategy. - Document - Gale Academic OneFile