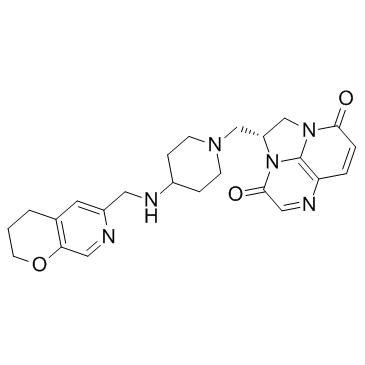
Antibiotics | Free Full-Text | Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy
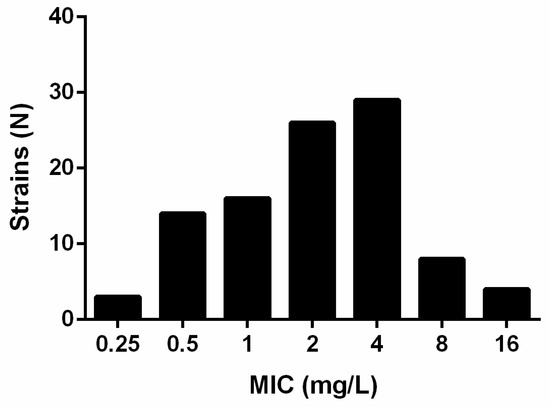
Antibiotics | Free Full-Text | Antibacterial Activity of the Novel Drug Gepotidacin against Stenotrophomonas maltophilia—An In Vitro and In Vivo Study

Dose Selection for a Phase 3 Study Evaluating Gepotidacin (GSK2140944) in the Treatment of Gonorrhoea | GSK

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK

Mechanistic and Structural Basis for the Actions of the Antibacterial Gepotidacin against Staphylococcus aureus Gyrase | ACS Infectious Diseases

Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants | SpringerLink
Phase 2a Pharmacokinetic, Safety, and Exploratory Efficacy Evaluation of Oral Gepotidacin (GSK2140944) in Female Participants wi

Gepotidacin (GSK2140944) Demonstrates Similar Safety and Pharmacokinetics in Adults and Adolescents (12 to ˂18 years) | GSK

Pharmacokinetics of Oral Formulations of Gepotidacin (GSK2140944), a Triazaacenaphthylene Bacterial Type II Topoisomerase Inhibitor, in Healthy Adult and Adolescent Participants | Antimicrobial Agents and Chemotherapy
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva
First Novel Antibiotic Since 20 Years, Gepotidacin Debuts To Treat Drug Resistance Urinary Tract Infections (UTIs) Amidst Growing Incidences! - Thailand Medical News