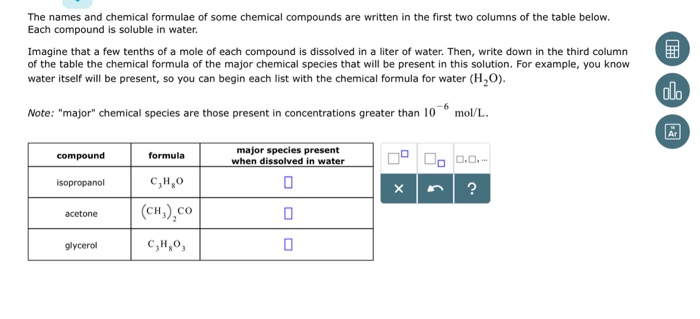
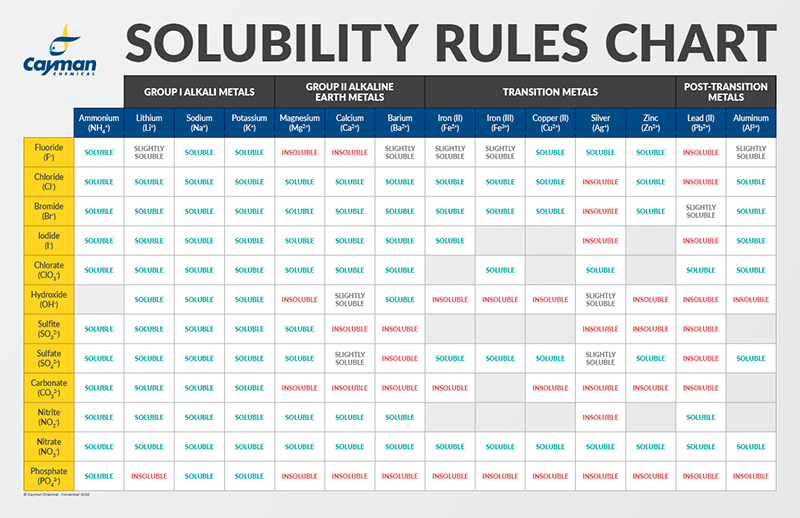
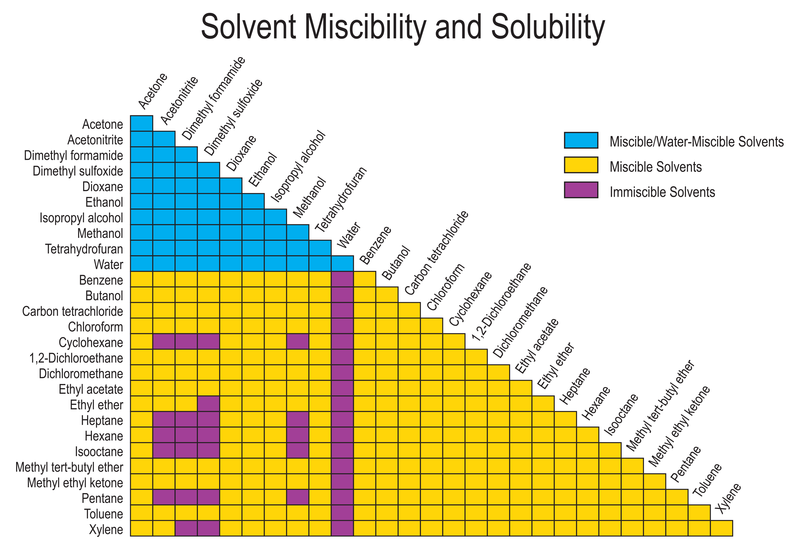
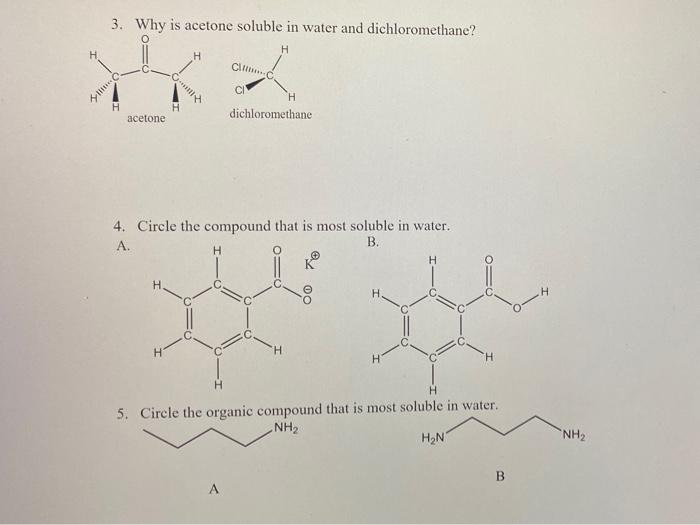
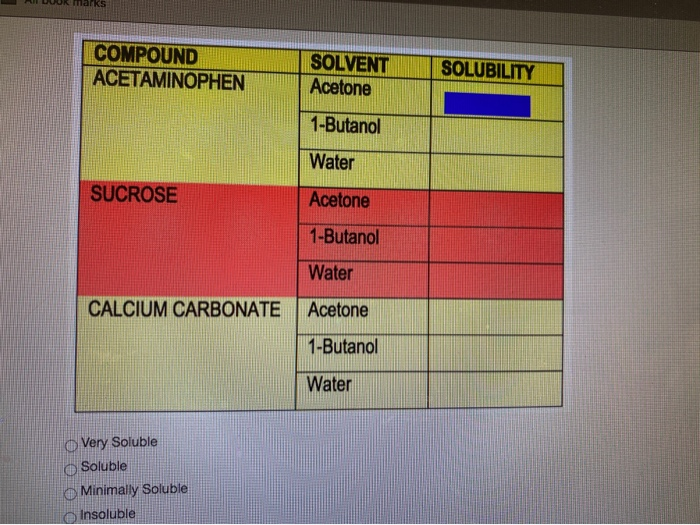
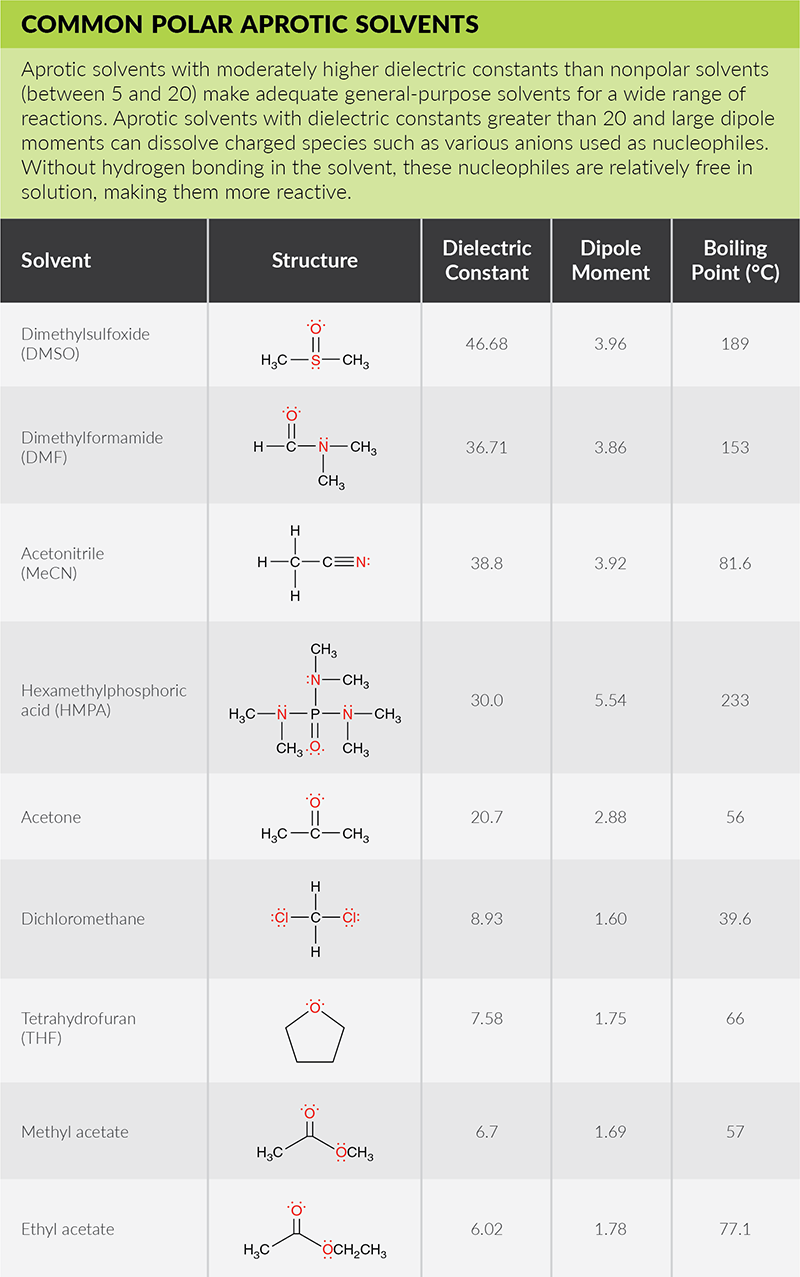
How would you explain the following observations?(i) BeO is almost insoluble but BeSO4 is soluble in water.(ii) BaO is soluble but BaSO4 is insoluble in water.(iii) LiI is more soluble than KI
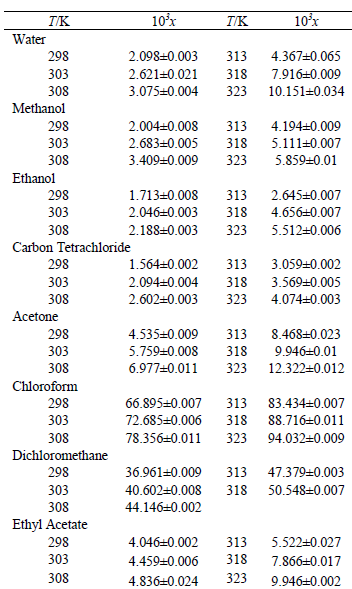
Solubility of caffeine in water, ethyl acetate, ethanol, carbon tetrachloride, methanol, chloroform, dichloromethane, and acetone between 298 and 323 K
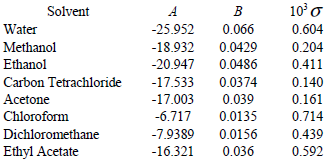
Solubility of caffeine in water, ethyl acetate, ethanol, carbon tetrachloride, methanol, chloroform, dichloromethane, and acetone between 298 and 323 K

Solubility of cetilistat in neat solvents and preferential solvation in ( acetone, isopropanol or acetonitrile) + water co-solvent mixtures - ScienceDirect

What is Lipid Lipids: insoluble in water, but soluble in organic solvents including diethyl ether, chloroform, methylene chloride, and acetone Amphipathic: - ppt download

Solubility. Soluble Sugar is soluble in water. Translation: Sugar dissolves in water. The solute particles are more attracted to the solvent than they. - ppt download
Carisoprodol is a centrally acting skeletal muscle relaxant. It is slightly soluble in water and freely soluble in alcohol, chloroform and acetone Stock Photo - Alamy
Carisoprodol is a centrally acting skeletal muscle relaxant. It is slightly soluble in water and freely soluble in alcohol, chloroform and acetone Stock Photo - Alamy