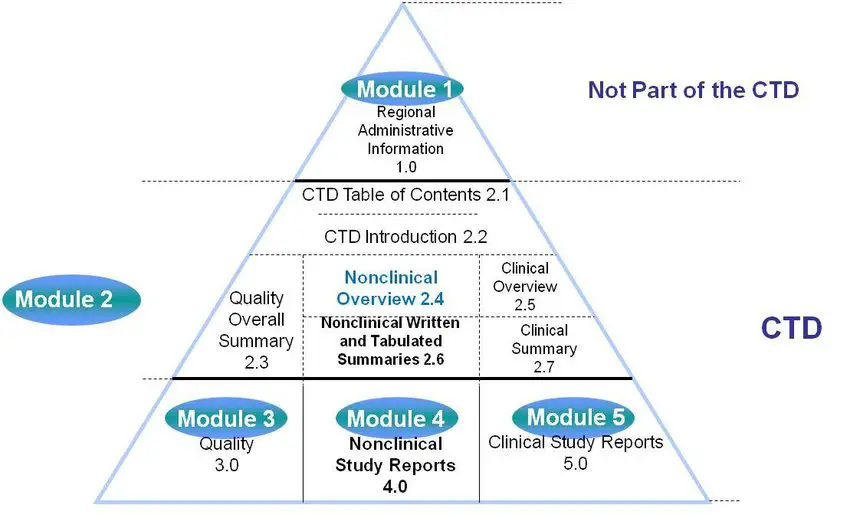
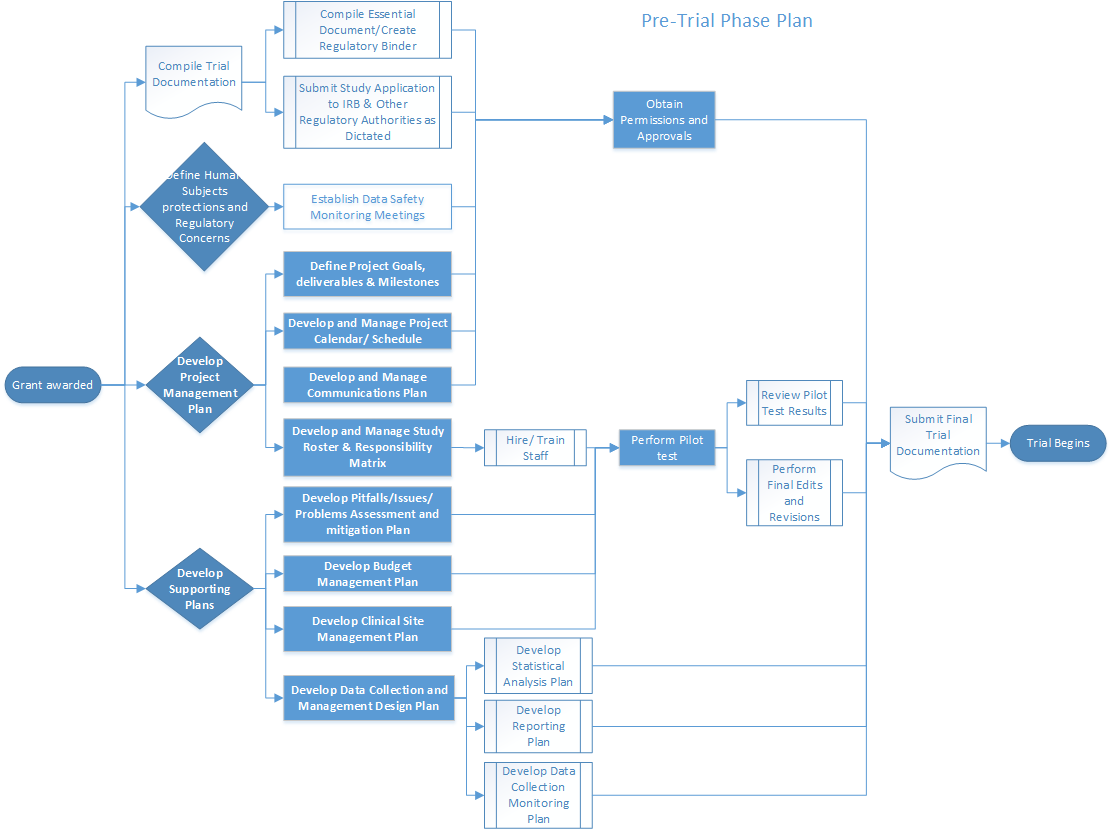
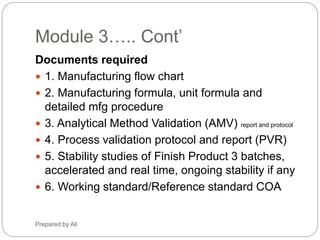
Nonclinical Information in the Common Technical Document: Opportunities for Content Reuse Peggy Zorn, MPI Research Susan Mattano, Pfizer, Inc. - ppt download

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

Benefits vs. Risks: Telling the Story in the Clinical Overview May be Changing - IMPACT Pharmaceutical Services, Inc.

Benefits vs. Risks: Telling the Story in the Clinical Overview May be Changing - IMPACT Pharmaceutical Services, Inc.